Another open surgery? I think not… Gastroenterology can seal the deal.
Filipa Lima1, Hugo Ribeiro1, Marta Gravito-Soares1,2, Elisa Gravito-Soares1,2, António Bernardes3, Pedro Figueiredo1,2
1. Gastroenterology Department, Hospitais da Universidade de Coimbra, Unidade Local de Saúde de Coimbra, Coimbra, Portugal
2. Faculty of Medicine, University of Coimbra, Coimbra, Portugal
3. Surgery Department, Hospitais da Universidade de Coimbra, Unidade Local de Saúde de Coimbra, Coimbra, Portugal
DESCRIPTION
A 56-year-old male patient with abdominal pain at upper quadrants and involuntary weigh loss (3% during one month) was diagnosed with adenocarcinoma of the gastroesophageal junction – Siewert II and underwent Ivor-Lewis subtotal esophagectomy (pTNM T2N0M0 HER2 negative, AJCC stage IB) after neoadjuvant chemotherapy using 5-fluorouracil plus leucovorin, oxaliplatin and docetaxel (FLOT). He had a previous history of smoking (39 pack years). On postoperative day two the patient started having dyspnea and chest pain. A thoracoabdominopelvic computed tomography (CT) was performed with no significant abnormalities or postoperative leak. Because of gradual worsening of those symptoms, right pleuritic chest pain and an increase of inflammatory markers, an emergent Gastroenterology consultation was requested on postoperative day five, suspecting of anastomotic dehiscence. An upper gastrointestinal endoscopy was performed revealing a gastroesophageal anastomotic leak of approximately 15 mm at 2 o’clock, near a suture thread. After a multidisciplinary discussion, it was decided to start endoscopic vacuum therapy (EVT). Initially, a single endoluminal sponge (Eso-Sponge® system, B. Braun, Melsungen AG, Melsungen, Germany) was endoscopically placed. However, due to an increase in the size of the dehiscence and the appearance of a second ipsilateral dehiscence, both allowed access to two independent large cavities (maximum largest diameter 38mm) (Fig. 1A), the EVT strategy was then switched to endocavitary using double sponges simultaneously, one in each cavity (Fig. 1B). The complete treatment included ten endoscopic sessions (six endocavitary and four intraluminal) totaling forty-nine days of EVT and 3.6 days between endocavitary sessions and 6.5 days between intraluminal sessions. Successful EVT treatment was confirmed endoscopically (Fig. 1C) and radiologically by thoracoabdominal CT with oral contrast (Fig. 2). The patient was discharged home 3 days later, after 59 days of hospitalization with no other complications and completed FLOT adjuvant chemotherapy. At 20 months follow-up, the patient remains asymptomatic with no recurrence of anastomotic leak or oncologic disease.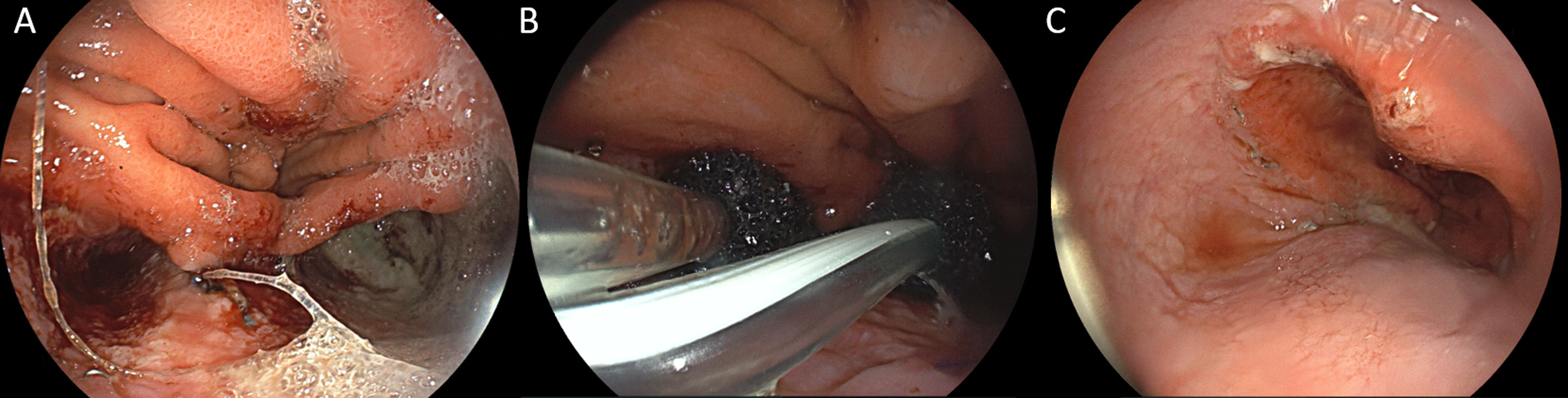
DISCUSSION
Despite the decreasing mortality and morbidity rates following esophagectomy, anastomotic leaks continue to occur in a significant number of patients. Possible treatments for post-esophagectomy include conservative management, endoscopic treatment, and surgery. There has been a progressive shift from surgical treatment to more conservative options (1). EVT represents an emergent endoscopic technique revealing to be a safe and effective approach for postoperative leaks after esophagogastric cancer surgery (2). When compared to self-expanding metal stents, recent studies have shown that EVT was associated with higher healing, shorter duration of treatment and stricture rate despite of higher number of endoscopic changes (3,4). The authors present a complex and challenging “spongy” case of an extensive postoperative dehiscence with 2 large cavities, in which EVT as monotherapy proved to be effective and safe, avoiding a longer delay in starting adjuvant therapy, as well as the need for surgical re-interventions in a critically ill patient and the associated morbidity and mortality. This negative pressure technique allowed the septic condition to be resolved by combining active drainage with healing by second intention. Surgery should be reserved for cases of endoscopic treatment failure or deterioration of clinical condition.
REFERENCES
1. Lee J, Jeon JH, Yoon SH, Shih BCH, Jung W, Hwang Y, et al. The optimal treatment strategy for postoperative anastomotic leakage after esophagectomy: a comparative analysis between endoscopic vacuum therapy and conventional treatment. J Gastrointest Surg. 2023;27(12):2899-2906.
2. Zhang CC, Liesenfeld L, Klotz R, Koschny R, Rupp C, Schmidt T, et al. Feasibility, effectiveness, and safety of endoscopic vacuum therapy for intrathoracic anastomotic leakage following transthoracic esophageal resection. BMC Gastroenterol. 2021;21:1-10.
3. Scognamiglio P, Reeh M, Melling N, Kantowski M, Eichelmann AK, Chon SH, et al. Management of intra-thoracic anastomotic leakages after esophagectomy: updated systematic review and meta-analysis of endoscopic vacuum therapy versus stenting. BMC Surg. 2022;22(1):309.
4. El-Sourani N, Miftode S, Bockhorn M, Arlt A, Meinhardt C. Endoscopic management of anastomotic leakage after esophageal surgery: ten-year analysis in a tertiary university center. Clin Endosc. 2022;55(1):58.


